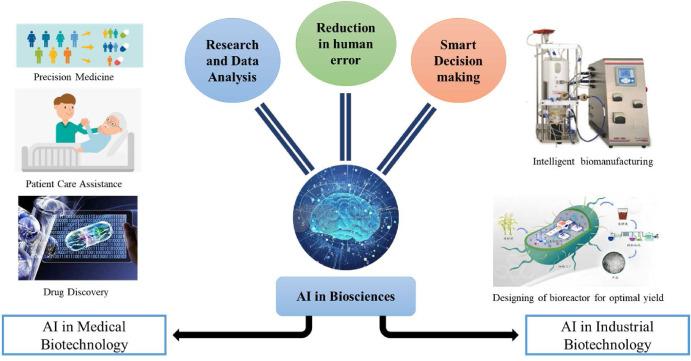
Artificial intelligence is moving from pilot projects to everyday practice across healthcare technology, as hospitals and vendors race to embed generative and predictive tools into clinical and administrative workflows. Electronic health record providers, imaging makers, and digital health startups are rolling out features to summarize notes, flag high‑risk patients, speed prior authorization, and triage scans-promising faster decisions and leaner operations amid staffing shortages and cost pressures.
The acceleration comes as regulators refine guidance for software that learns over time, cloud costs ease, and health systems seek measurable ROI after years of digital transformation. Yet the push is tempered by unresolved questions around data privacy, algorithmic bias, interoperability, and liability-issues now drawing heightened scrutiny from clinicians, patients, and policymakers. At stake is whether AI can translate headline‑grabbing advances into better outcomes and lower costs at scale. This article examines where adoption is taking hold, who stands to gain, and what could slow momentum in the year ahead.
Table of Contents
- Healthcare systems speed AI integration from pilots to production as regulators set clearer rules
- Imaging, triage, and clinical documentation show early gains while drug discovery and robotics lag
- Data quality and interoperability remain bottlenecks, prioritize shared standards, consent management, and rigorous validation
- Build clinician trust with explainable outputs, human in the loop oversight, and continuous post deployment auditing
- To Conclude
Healthcare systems speed AI integration from pilots to production as regulators set clearer rules
Hospital networks are moving from proof-of-concept to live clinical workflows as regulators publish clearer playbooks for safety, accountability, and change control. With guidance coalescing around risk classification, post-market surveillance, and predetermined change plans, CIOs report faster procurement cycles and tighter collaboration between compliance and engineering teams. Early production rollouts concentrate on high-volume use cases that are measurable and auditable, including:
- Imaging triage and workflow orchestration to prioritize critical studies and reduce turnaround times.
- Ambient clinical documentation that drafts visit notes and orders while preserving clinician oversight.
- Prior authorization and coding automation to cut administrative lag and denials.
- Capacity and throughput optimization for bed management, OR scheduling, and staffing.
- Patient engagement assistants that handle routine messages with clear escalation paths.
Operational guardrails are hardening alongside deployment velocity: health systems are standardizing model validation, instituting real‑time monitoring for drift, and requiring transparent change logs and rollback plans. RFPs now routinely demand HL7 FHIR compatibility, security attestations, bias testing, and clear data‑retention limits, while contracts codify performance SLAs and human-in-the-loop checkpoints. Executives say the next quarter will hinge on:
- Evidence of clinical benefit from multi-site studies and pragmatic trials, not just productivity gains.
- Lifecycle governance for continuous updates via vendor change plans and internal MLOps pipelines.
- Data protection at scale, including de-identification, access controls, and auditability.
- Workforce readiness-role-based training, scope-of-practice clarity, and escalation protocols.
- Reimbursement signals that determine which AI-enabled services can sustain long-term adoption.
Imaging, triage, and clinical documentation show early gains while drug discovery and robotics lag
Hospitals are reporting tangible wins in day-to-day operations, particularly in radiology reading rooms, emergency intake, and physician note capture. Imaging models embedded in PACS and workflow orchestration systems are accelerating report turnaround and flagging high-risk studies for earlier review under radiologist oversight. In parallel, intake tools are routing complex cases faster, and ambient clinical documentation is trimming clerical load while preserving billing detail and clinical nuance. Enterprise buyers cite human-in-the-loop governance, auditability, and seamless EHR integration as decisive factors turning pilots into production deployments.
- Well-bounded tasks win first: clear labels, tight guardrails, and objective metrics simplify validation.
- Workflow-native integration: PACS/EHR plugins and single sign-on reduce change management friction.
- Measurable outcomes: faster time-to-answer, better queue prioritization, and fewer rework loops.
- Procurement-friendly risk models: usage-based pricing and outcome guarantees speed approvals.
Momentum is slower in model-driven molecule design and hospital robotics. Discovery platforms are prolific at generating candidates, but wet-lab validation, tox profiles, and trial translation remain the gating steps, complicated by data silos and scarce negative examples. On the hardware side, unstructured clinical environments, safety certification, and integration with sterile workflows keep autonomous systems in pilot mode rather than widespread use. Investors and providers describe a near-term focus on “assist, not automate”: co-pilots that de-risk targets, tighten protocol hypotheses, or augment robotic perception, while larger bets on autonomy and new therapeutics timelines are paced by regulatory evidence and total-cost-of-ownership math.
Data quality and interoperability remain bottlenecks, prioritize shared standards, consent management, and rigorous validation
As AI pilots scale across hospitals and medtech, the chokepoint is no longer compute but the reliability and portability of clinical data. Fragmented EHR exports, device telemetry, imaging archives, and claims feeds still arrive in incompatible shapes, undermining comparability and audit trails. Executives and regulators increasingly demand tamper-evident provenance, harmonized vocabularies, and explicit patient permissions before models touch production workloads.
- Shared standards: Commit to FHIR R4/R5 endpoints, HL7 v2 bridges, and canonical terminologies (SNOMED CT, LOINC, RxNorm, ICD-10) with documented mappings and versioning.
- Consent management: Centralize granular, revocable consent with OAuth2/SMART scopes, data minimization, and machine-readable policy logs to ease cross-institution collaboration.
- Rigorous validation: Enforce dataset shift detection, site-stratified holdouts, external validation, and post-deployment surveillance with explainability and drift alerts.
- Lineage & governance: Maintain provenance graphs, immutable hashes, and change control for schemas and code to satisfy audit and reimbursement requirements.
Organizations moving fastest are operationalizing these practices through data contracts with vendors, pre-production “clinical sandboxes,” and continuous QA that blocks promotion if quality thresholds slip. Payers and health authorities are tying funding to transparent performance reporting-bias checks across demographics, error analysis by care setting, and independent replication-pushing AI suppliers to publish model cards, support federated evaluation where data cannot move, and ship APIs that honor consent at query time as rigor becomes a market differentiator.
Build clinician trust with explainable outputs, human in the loop oversight, and continuous post deployment auditing
Hospitals racing to scale machine learning are finding that adoption hinges on transparency clinicians can act on. Teams are surfacing model reasoning at the point of care, pairing predictions with context clinicians recognize, and making the provenance of inputs explicit. That means showing not just a score, but why it moved, how confident the system is, and when it should be sidelined. The result: faster bedside decisions without abandoning professional judgment.
- Plain-language rationales: concise explanations that map to clinical concepts and guidelines.
- Top contributing factors: ranked features with direction and magnitude, plus data lineage for source verification.
- Confidence and guardrails: uncertainty bands, “do-not-use” thresholds, and contraindication flags.
- Comparators: patient-specific baselines and cohort benchmarks to calibrate risk.
- Traceable links: citations to protocols, literature, and timestamped inputs within the EHR.
Equally critical is operational oversight: real clinicians remain decision-makers, with structured review, documented overrides, and full audit trails. Post-market monitoring now mirrors safety practices in other regulated domains, with live dashboards tracking accuracy, drift, and equity, and with formal change control for every model update. Organizations that institutionalize these controls are reporting fewer alert dismissals and higher acceptance at the unit level.
- Review workflows: queueing for second reads, escalation rules, and required justification on override.
- Safety-first releases: shadow mode, canary rollouts, and automatic rollback tied to performance SLAs.
- Continuous auditing: real-time drift and bias checks, error tracing, and CAPA documentation.
- Lifecycle governance: versioned model cards, change logs, and periodic revalidation against fresh cohorts.
- Feedback loops: clinician annotations feeding retraining pipelines via secure, de-identified pathways.
To Conclude
As AI tools move from pilots to production, the next phase in healthcare will be defined less by demos and more by validation. Health systems and vendors alike face a narrowing set of tests: measurable outcomes, interoperability with entrenched EHRs, clear governance, and airtight security. Workforce pressures and administrative costs are pushing adoption, even as data quality, bias, and privacy remain points of friction and regulators signal closer scrutiny.
With funding steady and early case studies multiplying, the debate is shifting from whether to integrate AI to how-and how safely-to deploy it at scale. The winners will couple clinical evidence with accountable rollout and transparent guardrails. Over the coming year, the market will determine whether early gains translate into durable value for patients, providers, and payers across the care continuum.